
In an electrochemical cell, the electrolysis process takes place. For this performing process, we require two main components such as an electrolyte solution and types of electrodes.
Basically, an electrode is classified into two types. One is an anode and another is a cathode.
The anode and cathode are immersed in an electrolyte solution to generate DC power. Depending on the cells, both electrodes have different working functions.
Let’s dive into what is the basic difference between anode and cathode in tubular form.
Anode vs Cathode
| # | Content | Anode | Cathode |
| 01 | [Defination] What is the anode and cathode? | The electrode that carries conventional current from the positive terminal of the battery to the negative terminal is called an Anode. | The electrode that carries electric current from the negative terminal of the battery to the positive terminal is called a Cathode. |
| 02 | Representation of Anode & Cathode | The positive (+) terminal of the electrode shown in the battery is the anode. | The negative (-) terminal electrode shown in the battery is the cathode. |
| 03 | Called | Sometimes, an anode (positive charge) is called as an electron donor. | A cathode (negative charge) is called an electron acceptor. |
| 04 | What type of reaction occurs at the cathode & anode? | In both galvanic and electrolytic cells, an oxidation reaction occurs at the anode. | In both galvanic and electrolytic cells, a reduction reaction occurs at the cathode. |
| 05 | Anode & cathode in electrolyte cells | It has excess positive charges in electrolyte cells. | It has excess negative charges in electrolyte cells. |
| 06 | Anode & cathode in galvanic cell | An anode becomes a cathode (negative charge) in a galvanic or voltaic cell. | A cathode becomes an anode (positive charge) in a galvanic or voltaic cell. |
07. Representation of anode and cathode symbol in battery.
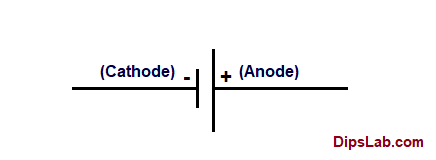
Note: The charge positions inside the battery on electrodes (anode or cathode) can vary. It means that charge position depends on the types of electrical cells.
These are 7 points that covered anode vs cathode. Also, I have described the difference between the galvanic cell and electrolytic cell.
If you have any queries regarding the difference between anode and cathode, you can ask in the comment.
Read related some differences:
- Electrical vs Electronics
- Alternating current vs Direct current
- Battery vs Capacitor
- Power vs Energy
- Active power vs Reactive power
- Electrical circuit vs Magnetic circuit
Thanks for Reading!